ABOUT THE PROJECT
NEOLIVER is a Horizon Europe research and innovation project developing world’s first autologous bioprinted liver which will be validated in large animal models. Our ultimate goal is to produce dense, vascularized bioprinted liver constructs suitable for clinical use as an Advance Therapy Medicinal Product (ATMP).
NEOLIVER will tackle key technological challenges and barriers in whole organ engineering by merging two bioprinting technologies and exploring different innovation routes.
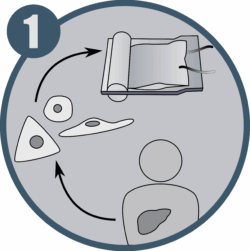
Large-scale production protocols for cell mass expansion, differentiation, and cryopreservation
|
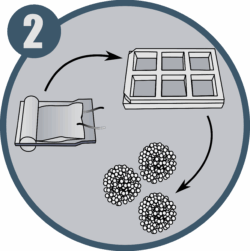
Form multicellular spheroids that recapitulate the complexity of native liver tissue
|
|
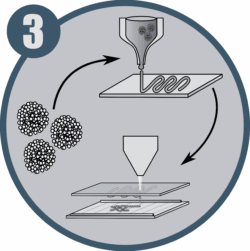
Cell-degradable hydrogels development for spheroid encapsulation and bioprinting using LIFT technology
|
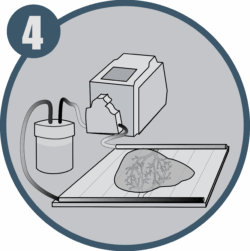
Construct microvascularization and maturation in a perfusion bioreactor
|
|
Automated GMP-conform manufacturing
|
Surgical implantation and evaluation in immunodeficient pig model
|
|
Surgical implantation and evaluation in immunodeficient pig model
|
||